Logohu në mjekët.al
- MESCALINE
- LSD – LYSERGIC ACID DIETHYLAMIDE
- PSILOCYBIN
- PCP - PHENCYCLIDINE
- KETAMINE
- GHB - GAMMA-HYDROXYBUTYRATE
HALLUCINOGENS
Psychedelic substances are characterized by their ability to cause changes in a person's perception of reality. People who use psychedelic drugs often claim to see images, hear sounds, and feel sensations that seem real, but do not exist.
In the past, plants and mushrooms containing psychedelic substances were abused. Today, these psychedelic substances are synthetically produced to provide a high effect.
Psychedelic substances include:
- Mescaline
- LSD – Lysergic acid diethylamide - 400 times stronger than mescaline.
- Psilocybin
- PCP – Phenylcyclohexyl piperidine, also known as Phencyclidine
- Ketamine - known as Special K, designed as a fast-acting drug
- GHB - gamma-hydroxybutyrate
- GBL - gamma-butyrolactone, a drug that converts in the body to GHB.
The traditional use of Morning glory seeds by indigenous Mexicans in the USA was first described by Richard Schultes, in 1941, in a brief report documenting their use since the time of the Aztecs.
A further study was published in 1960, when Don Thomes MacDougall reported that the seeds of the Ipomoea tricolor plant were used as a sacrament (sacred) by some Zapotec tribes, sometimes together with the seeds of the Rivea corymbosa plant, another species, which has a similar chemical composition, with lysergol instead of ergometrine.
The statues found have confirmed the use of these plants since ancient times.
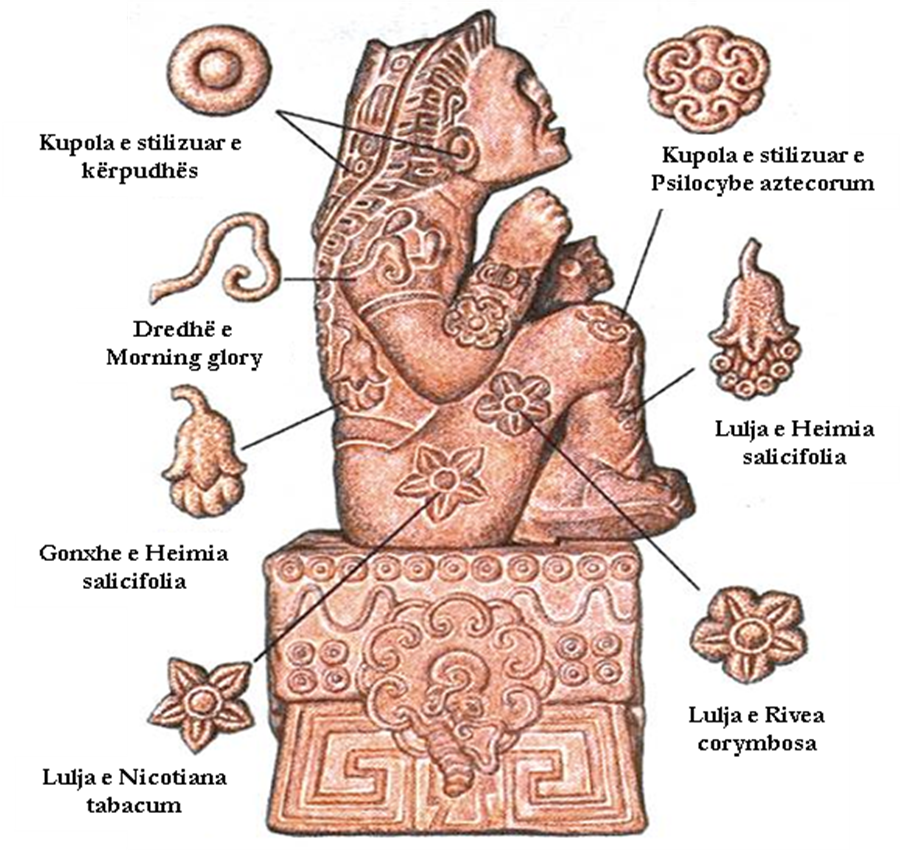

Below we are showing some of the plants that contain psychotropic substances, which have been stylizedly carved into the found statues.

Heimia salicifolia (Sun-Opener, Sinicuichi)

Rivea corymbosa (Ololiuqui)

Morning glory

Psilocybe aztecorum
MESCALINE
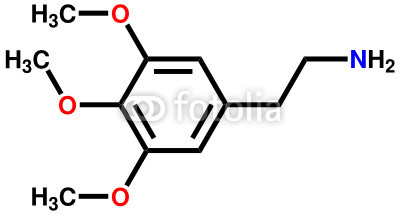
2-dimensional chemical structure of mescaline

3-dimensional chemical structure of mescaline
- Appearance: White crystalline powder, tablets or capsules
- Formula: C11H17NO3
- Molar mass: 211.257 g/mol
- Synonyms: 3, 4, 5 – trimethoxyphenethylamine
- Melting point: 180 °C (Dehydrated Sulfate)
- Routes of administration: Oral, Intravenous
- Plant origin: Peyote - a plant grown in Latin America

THE PLANT
Mescaline is an extract of peyote. The “Peyote” plant (Echinocactus williamsii), also known as Lophophora williamsii, belongs to the Cactus family.
It is a small, spineless cactus with very slow growth, reaching a maximum diameter of 15 cm and a height of about 10 cm, more or less rounded, bulbous, the surface of which is divided into several stacked lobes bearing a tuft of hairs.
The plant has a carrot-shaped root. In the natural environment, flowers appear in April. A single flower, red, yellow, or white, blooms in the center of the cactus and produces a red fruit after pollination.


The region of origin of peyote is the southern Texas and the central Mexican plateau. Today, the plant has spread in Mexico (tribes Huichols, Tarahumaras, Coras, etc.) and in the USA (tribes Comanches, Kiowas, Navajos, etc.) among about fifty different tribes.
The consumption of peyote is associated with divine, therapeutic, and religious practices.
Peyote has a bitter taste.
The need to chew on the plant pieces for a long time sometimes provokes gagging, up to vomiting.
As pure mescaline, peyote provokes rich visual hallucinations. The plant is also called “The Plant that Makes the Eyes Marvel” and during the consumption rituals, it is supposed that one communicates with the gods.
However, the extended gathering of peyote makes it a species threatened by extinction.
Peyote contains fifteen alkaloids, in which the most important is mescaline. The plant also contains some alkaloids that have sedative effects such as anhalonine, anhalonidine, and peyotline, or toxic effects like lophophorine.
Also, mescaline is found in another cactus, the San Pedro cactus or Trichocereus pachanoi.

San Pedro Cactus (Trichocereus pachanoi)
HISTORY
Peyote has been used around 5700 years ago, in Mexico, by the Native Americans, Aztecs. The other cactus that contains mescaline, such as San Pedro, has a long history of use in South America, from Peru to Ecuador.
In the traditional preparations of peyote, the top of the cactus is cut at ground level, leaving the large roots embedded to grow new 'heads'. These 'Heads' are then dried to form disc-shaped "buttons" (mescal button). The buttons are chewed to produce effects or soaked in water to drink. However, the taste of the cactus is bitter, so contemporary users often grind it into a powder and encase it in capsules to avoid its taste. The usual human dose is 200 - 400 milligrams of mescaline sulfate or 178 - 356 milligrams of mescaline hydrochloride. An average 76 mm (3 inch) “button” contains 25 mg of mescaline.

Peyote "Mescal button"
Mescaline was isolated and identified for the first time in 1897 by the German chemist Arthur Heffter and was synthesized for the first time in 1919 by the Austrian chemist Ernst Späth.
Around the year 1918, the peyote cult appeared in the USA.
In 1950, Professor Delay's team showed that mescaline provokes a loss of personality, curiously similar to what we encounter in various schizophrenias.In 1955, English politician Christopher Mayhew took part in an experiment for BBC Panorama, in which he ingested 400 mg of mescaline under the supervision of psychiatrist Humphry Osmond. Although the program was rated as very controversial and ultimately allowed to be seen, Mayhew rated the experience, calling it “The most interesting thing I have done”.
MODE OF CONSUMPTION
Mescaline is responsible for the hallucinogenic effects of peyote. Mescaline:
- Is taken orally;
- Is smoked;
- Is injected.
Mescaline is active at doses of 300 to 500 mg when taken orally.
The effects begin to manifest after 30 - 40 minutes following ingestion with a state of euphoria. They develop progressively and can last from 10 to 18 hours.
Approximately 90% of samples believed to be mescaline, analyzed in toxicology laboratories, contained PCP, LSD, amphetamine, aspirin, and even strychnine.
SHORT-TERM EFFECTS
Mescaline is a sympathomimetic: it slightly increases heart rate, blood pressure, sweating, and salivation. It is characterized by psychedelic-type hallucinations, generally visual, with particularly bright colors, but also auditory. Synesthetic hallucinations (coloring of sounds) and feelings of empathy are continuous, and mystical episodes are not rare.
Its short-term effects are:
- Pupil dilation;
- Fever;
- Alternation of perception and mood;
- Disorientation;
- Alteration of immediate memory;
- Impaired concentration.
LONG-TERM EFFECTS
The long-term effects of mescaline are:
- Anxiety;
- Emotional disturbances;
- Paranoia;
- Dilation of the pupils;
- Abnormal visual perception;
- Nausea and vomiting.
A few days' interval after taking mescaline is enough for the user to regain all their sensitivity.
Chronic users of mescaline can become psychologically dependent.
With high doses, mescaline can cause:
- Headaches;
- Dry skin;
- Drop in blood pressure;
- Slowing of heart rate and breathing.
Due to the higher availability of other hallucinogens on the black market, mescaline is not a subject of abuse.
Various derivatives more active than mescaline have been synthesized (escaline, proscaline, thiomescaline, trimethoxyamphetamine, etc), but they are appearing less frequently on the black market.
LSD – LYSERGIC ACID DIETHYLAMIDE
GENERAL INFORMATION
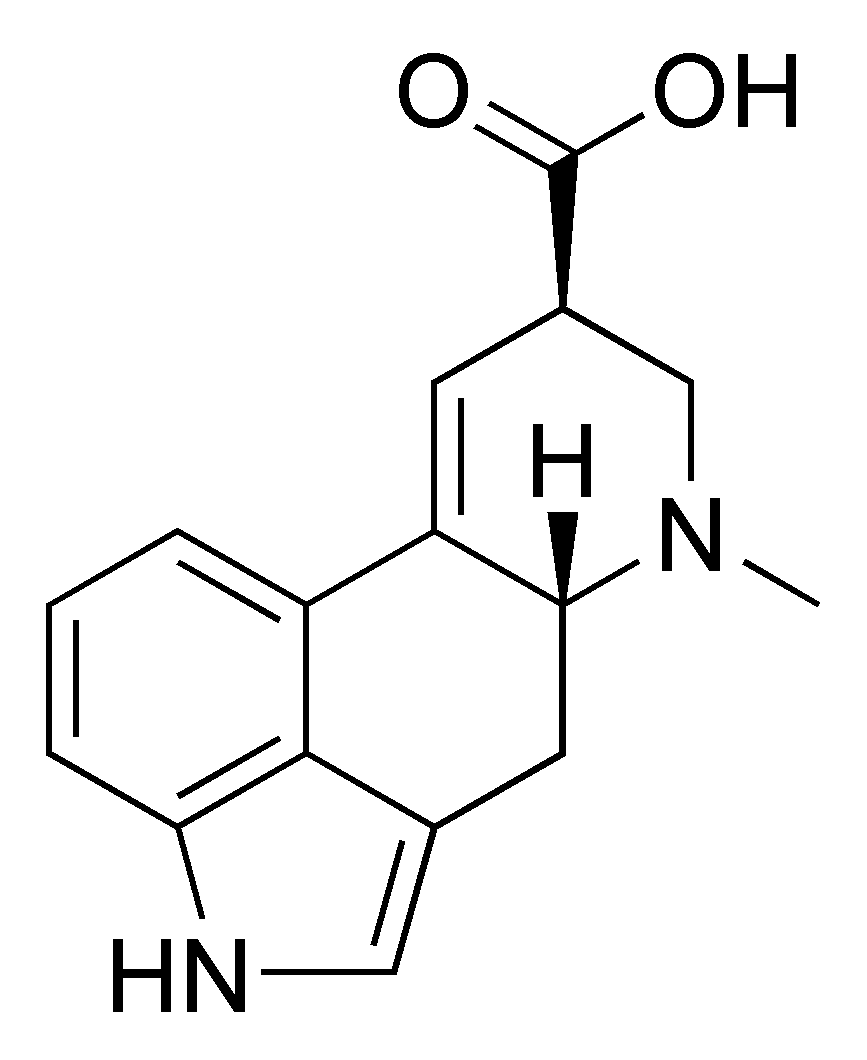
2-dimensional chemical structure of LSD
3-dimensional chemical structure of LSD
- Molecular formula: C20H25N3O
- Molecular weight: 323.4 g/mol
- Melting temperature: 80-85 °C
- Synonyms: LSD; LSD-25; lysergide; D-lysergic acid diethylamide; N,N-diethyl-D-lysergamide
- Physical appearance
- Typically, LSD is produced from tartaric acid, which is colorless, odorless, soluble in water. The most common doses obtained are in the form of "blotters" or "paper squares", that is, absorbent paper decorated with distinctive drawings and perforated to enable division into squares (usually measuring 7 mm), with a single dose. Each sheet generally contains at least 100 doses.
- Also, LSD can be found in the form of small tablets ("microdots") with a diameter of 2-3 mm.
- LSD can be found in the form of thin gelatin squares ("window panes") or in capsule form.
- Occasionally, we can find LSD solution diluted in water or alcohol. LSD is sensitive to light when it is in solution.
The abbreviation LSD comes from the German language name: Lyserg Säure Diethylamid (D-lysergic acid diethylamide). Lysergide belongs to a family of indolealkylamines that includes a large number of substituted tryptamines such as psilocin (found in "magic" mushrooms) and N,N-dimethyltryptamine (DMT).




THE PLANT
Most methods for producing LSD use lysergic acid as a precursor. Also, lysergic acid itself is often produced in clandestine laboratories using ergometrine or ergotamine tartrate (a salt of tartaric acid) as a starting material.
Ergotamine is naturally present in ergot (Claviceps purpurea). Ergot is produced by a low-level fungus (Claviceps purpurea), which grows parasitically on rye and, to a lesser extent, on other species of cereals and wild grasses.
Ergometrine (also known as ergonovine), ergotamine, and lysergic acid are listed in Schedule I of the Annex to the 1988 United Nations Convention against Illicit Traffic in Narcotic Drugs and Psychotropic Substances.
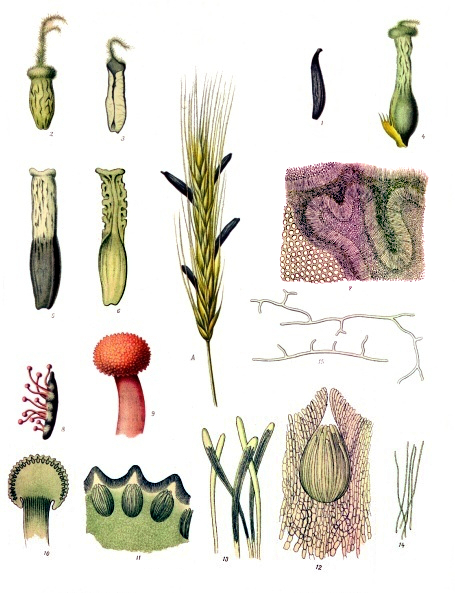


Ergot of rye (Secale cornutum) is widely used in medicine.
Ergot was first mentioned in the early Middle Ages as the cause of massive poisoning outbreaks that affected thousands of people at a time. The disease appeared in two characteristic forms, a gangrenous form (ergotismus gangraenosus) and a convulsive form (convulsivus ergotismus).
Popular names for ergotism such as: “the evil of fire” (“mal des ardents”), “false holy hope” (“ignis sacer”), “holy fire” (“heiliges Feuer”) or “St. Anthony’s fire” (“St. Anthony’s fire”) refer to the gangrenous form of the disease.
The patron saint of ergotism victims was Saint Anthony, and it was primarily the Order of Saint Anthony that treated these patients.
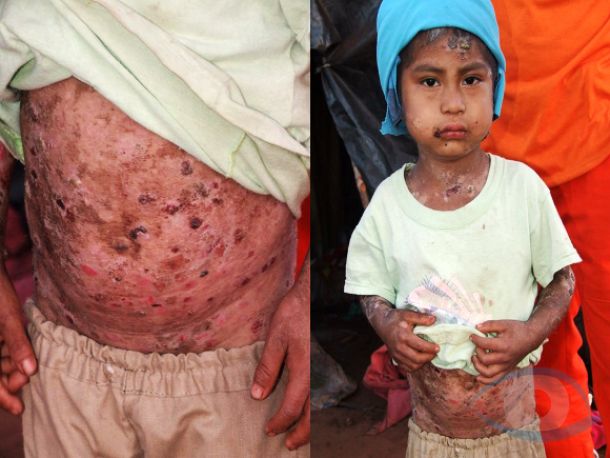
Gangrenous form of ergotism
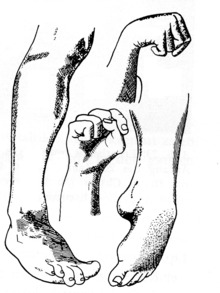
Convulsive symptoms of ergotism
Until many years ago, poisonous outbreaks of ergots approaching epidemic proportions have been recorded in most European countries, including certain areas of Russia. However, in the seventeenth century, it was discovered that the content of ergot in bread was the cause of poisonings. This, along with advances in agriculture, significantly reduced the frequency and level of ergotism epidemics. The last major epidemic occurred in certain areas of southern Russia in the years 1926-1927.
Because of ergot, the infamous witch trials are well-known, which began in the spring of the year 1692, after a group of young girls in the Salem village, in Massachusetts, claimed they were possessed by the devil and accused some local women of witchcraft.
The first mention of the medical use of ergot, as a sudden birth medication, is found in the records of the Frankfurt city physician Adam Lonitzer, in the year 1582. Although ergot had been used since ancient times until the year 1808 by midwives, it had not gained recognition as a medication in academic medicine. However, the use of ergot for these purposes does not continue, because the uncertainty of dosing leads to uterine spasms and risks to the child.
The beginning of the 1930s brought a new era in scientific research for ergot, starting with the determination of the chemical structure of the main chemically active agents, ergot alkaloids. Recently, W. A. Jacobs and L. C. Craig of the Rockefeller Institute in New York succeeded in isolating and characterizing the common core for all ergot alkaloids. They named it lysergic acid.
HISTORY
In the late 1930s, Albert Hoffman was working in the pharmacological department of the Sandoz laboratory in Basel, Switzerland. Hoffman was studying derivatives of lysergic acid with different reagents to produce the corresponding amides, the corresponding anhydrides, the corresponding esters, etc. One of these derivatives was diethylamide, produced by adding the group - N(C2H5)2, and was named LSD-25. The new substance (synthesized in 1938) did not appear to have any particularly useful medicinal properties, although the study report noted, incidentally, that “experimental animals became restless during anesthesia”. Over the next five years, nothing more was heard about the substance LSD-25.
In the spring of 1943, Hoffman repeated the synthesis of LSD-25.
Due to its hallucinogenic effects, LSD was widely adopted by the “hippy” culture of the 1960s.
“The Beatles” wrote a song, “Lucy in the Sky with Diamonds”, that allegedly described the psychedelic effects of LSD.
Although LSD is relatively non-toxic and not addictive, various governments around the world have outlawed it, following reports of a number of fatal accidents.
MODE OF CONSUMPTION
LSD is consumed orally. Blotter doses are placed on the tongue, where the drug is quickly absorbed. Tablets or capsules are swallowed. LSD is not absorbed through dry skin.

SHORT-TERM EFFECTS
By experimenting on himself, Hoffman described the effects as follows:
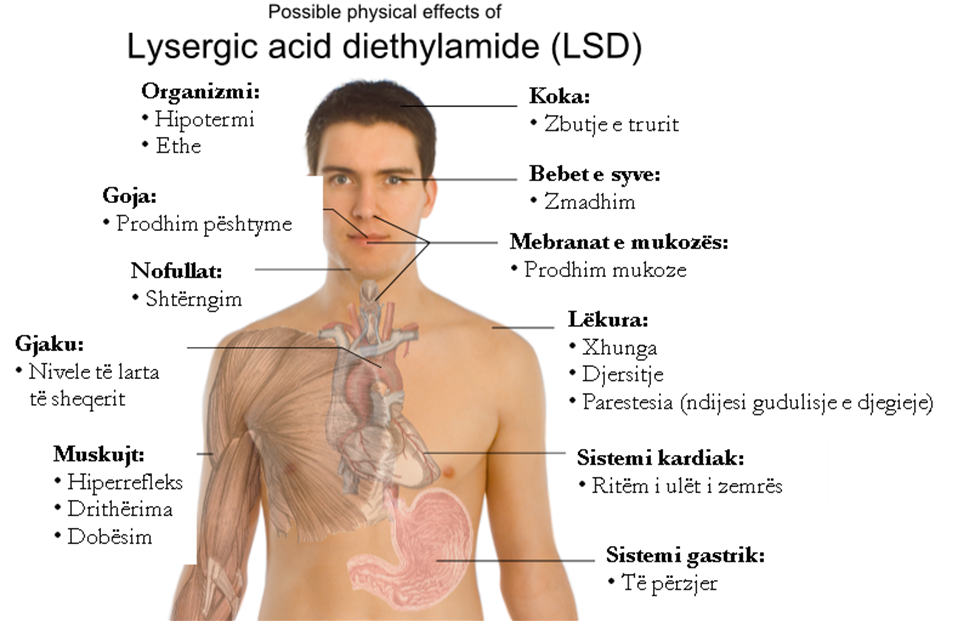
"17:00: Dizziness begins, feeling of anxiety, visual disturbances, symptoms of paralysis, desire to laugh. … Everything in my field of vision wobbled and distorted as if I were looking through a curved mirror. I also had the sensation of being unable to move from the spot. …The dizziness and the feeling of nausea became so strong over time that I could no longer stand on my feet and had to lie down on a sofa. Everything around me had now transformed in the most horrifying ways. Everything in the room spun around, and familiar objects and pieces of furniture took on grotesque, threatening shapes. They were in constant motion, animated, as if driven by an internal restlessness."POSSIBLE PHYSICAL EFFECTS
PSILOCYBIN
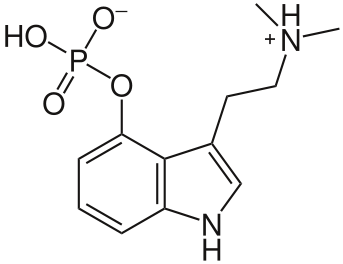
2-dimensional chemical structure of Psilocybin
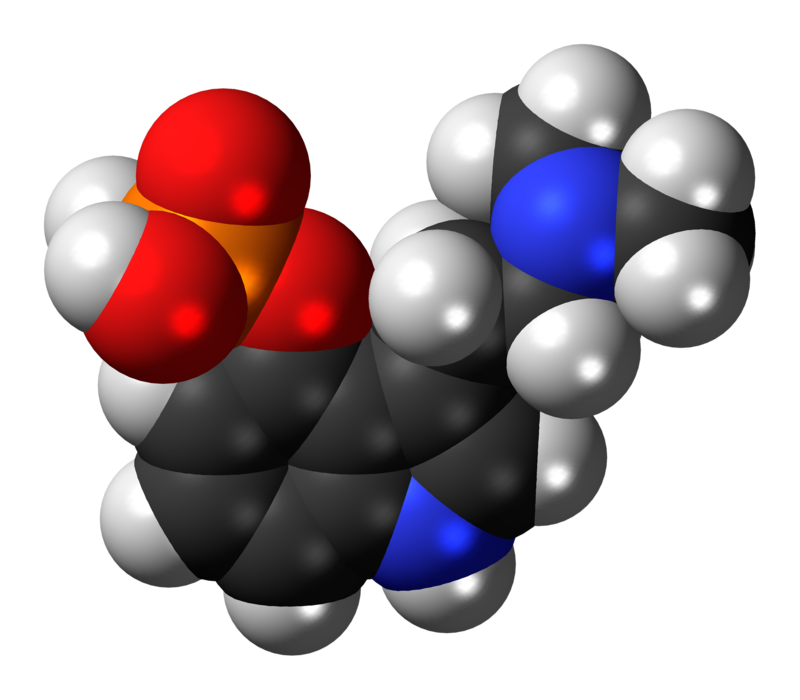
3-dimensional chemical structure of Psilocybin
- Formula: C12H17N2O4P
- Molar mass: 284.25 g/mol
- Melting point: 220-228 °C
- Solubility: Soluble in water, soluble in methanol, slightly soluble in ethanol, very slightly soluble in chloroform and benzene
- Ways of consumption: Oral, intravenous
- Half-life: 160 ± 60 minutes
Psilocybin is a mild hallucinogen (entheogenic, psychedelic) belonging to the tryptamine family. In its pure state, it is a white crystalline substance, but it can be sold in the form of fresh or dried mushroom pieces, small brown mushroom pieces.
Proponents of its use consider it an entheogen and a tool for completing various types of miraculous practices (transcendence), including meditation practices, psychonautics, and psychedelic psychotherapy.
As soon as it is chewed, psilocybin is very quickly metabolized into psilocin, which then acts as a partial agonist on the 5-HT2A and 5-HT1A serotonin receptors in the brain.
The mood-altering effects of psilocybin typically last 3 to 8 hours, however, under the influence of psilocybin, the effects may seem to last longer, as the drug can distort the perception of time.
The possession and, in some cases, use of psilocybin or psilocin is prohibited in most countries of the world.



PLANTS
Psilocybin is a natural component found in varying concentrations in more than 200 species of mushrooms. The most well-known species are:
- “Psilos” mushrooms (psilocybe semilanceata)
- Mexican mushrooms (psilocybe cubensis)
- Hawaiian mushrooms (psilocybe cyanescens)
Known as psilocybin mushrooms these are collectively called “boomers”, “sacred mushrooms”, “magic mushrooms”, or simply “shrooms”.
Mushroom spores do not contain psilocybin or psilocin. Mushroom caps tend to contain most of the psychoactive substances, more so than the stalk.
New species, the small-sized mushrooms, have a higher concentration of alkaloids and have a sweeter taste than the large mushrooms, those that are ripe.
In general, the content of psilocybin in mushrooms is very variable (about 0.5% - 2% of dry weight) and depends on the species (type), growth, drying conditions, and size of the mushrooms. Mature mycelium contains a good amount of psilocybin, while young mycelium (freshly sprouted from spores) does not contain significant amounts of alkaloids.
Many species of mushrooms that contain psilocybin also contain small amounts of psilocybin analogs, baeocystine and norbaeocystine.
Most species of mushrooms containing psilocybin turn blue when manipulated or damaged due to the oxidation of phenolic components. This reaction, however, is not a definitive method of identification or of determining the mushroom's potency.

Psilocybe semilanceata

Psilocybe cubensis

Psilocybe cyanescens

Amanita muscaria
There are evidences suggesting that psychoactive mushrooms have been used by humans in religious ceremonies for thousands of years. Mural paintings dating back to 9,000 - 7,000 BC, found in the Sahara Desert in southeastern Algeria depict creatures with antlers dressed as dancers, adorned in garments decorated with geometric figures and holding objects like mushrooms. Parallel lines extend from the mushroom shapes in the center of the dancers' heads. 6,000-year-old paintings, discovered near the Spanish city of Villar del Humo illustrate some mushrooms that have been preliminarily identified as the species Psilocybe Hispanica, a hallucinogenic species native to the area.
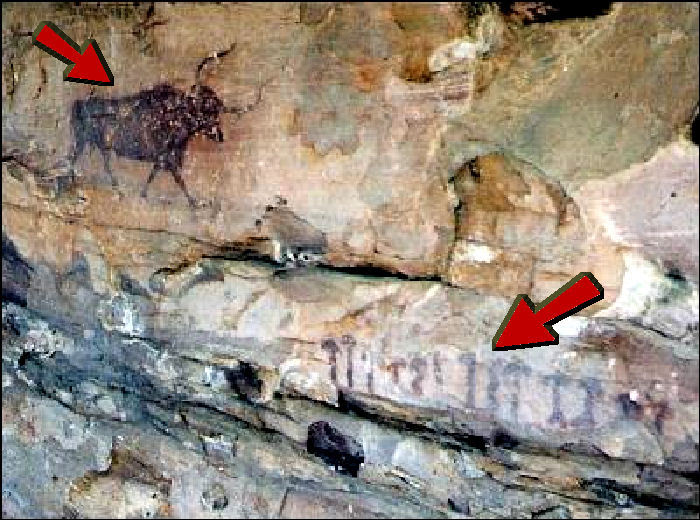
6,000-year-old paintings, discovered near the Spanish city of Villar del Humo
Archaeological objects from Mexico, as well as the so-called “mushroom stones” of the Maya tribes of Guatemala, have also been interpreted by some researchers as evidence of ritual and ceremonial use of psychoactive mushrooms in the Maya and Aztec cultures of Central America. In the Nahuatl language, the language of the Aztecs, mushrooms were called teonanácatl, or “flesh of God”.
After the arrival of Spanish explorers in the New World, in the 16th century, chroniclers reported the use of mushrooms by the locals for ceremonial and religious purposes. According to the Dominican monk Diego Durán in the book “The History of the Indians of New Spain” (published in 1581), mushrooms were consumed in celebrations held on the occasion of the enthronement of the Aztec emperor Moctezuma II in the year 1502.

Franciscan friar Bernardino de Sahagun wrote about evidence of mushroom use in his Florentine Codex (published in the years 1545-1590) and described how some merchants would celebrate after returning from a successful business trip, by consuming mushrooms, to induce astonishing visions.
After the defeat of the Aztecs, the Spaniards banned traditional religious practices and rituals that they considered "pagan worship", including the ceremonial use of mushrooms. For the next four centuries, the Indigenous people of Central America hid from the Spanish authorities their use of entheogens.
Although some psychedelic mushrooms are found in Europe, there is little documentation on the use of these species in European history. Flemish botanist Carolus Clusius (1526-1609) described bolond gomba (the mushroom that drives you mad), used in rural areas of Hungary, to prepare the “love elixir”. English botanist John Parkinson compiled details about a “laughing mushroom” in his herbal manual Theatricum Botanicum, in 1640.
The first reliable documented report of intoxication with Psilocybe semilanceata (the most common and widespread psychedelic mushroom in Europe) involves a British family in 1799, which had prepared a meal with mushrooms that it had gathered in London's Green Park.
In 1957, the American ethnologist R. Gordon Wasson and his wife published in Life magazine the article "In Search of the Hallucinogenic Mushrooms", where they described the experiences of hallucinatory experiences during the early religious rites of the Indians in a lost village of Mexico. They were later joined on a subsequent expedition by the French mycologist Roger Heim, director of the National Museum of Natural History, where he made possible the identification of many mushrooms such as those of the Psilocybe species. Heim successfully cultivated the mushroom in France and sent samples to the Swiss chemist Albert Hofmann for analysis. Hofmann, who had already discovered and experimented with LSD, was the first to recognize the importance and chemical structure of the pure components he called psilocybin and psilocin.
HISTORY
Surrounded by a group of scientific researchers who were able to isolate and identify the components of the Psilocybe mexicana mushroom, Hofmann was assisted in the discovery process by his willingness to chew mushroom extracts.
In the early 1960s, Harvard University became the grounds for psilocybin trials, thanks to the efforts of Timothy Leary and his assistant Richard Alpert (now known as Ram Dass). Leary was able to obtain synthesized psilocybin.
In the early 1960s, a large number of experiences showed positive results in the use of psilocybin in psychiatric clinics, using “psycholytic therapy” (psychotherapy related to the controlled use of psychedelic substances).
The 1970s would witness the emergence of psilocybin as the “entheogen of choice”. But the hysteria of the LSD era engulfed psilocybin along with it in the Schedule I of controlled substances in 1971.
WAYS OF CONSUMPTION
Consumed: reduced to powder, fresh, dried, in sauce form, in tea form. Generally by being absorbed through the nose or swallowed through the mouth. Very rarely smoked, because the effects are weaker in this case.
Dosage: the concentration in the active principle varies greatly. The average doses of dried mushrooms (multiplied by ten for fresh mushrooms) are:
- Type “psilocybin”
- Light: 0.5 - 0.8 g
- Moderate to strong: 1.0 - 2 g maximum
- Type “Hawaiian”
- Light: 0.3 - 0.5 g
- Moderate to strong: 0.5 - 1 g maximum
- Type “Mexican”
- Light: 0.3 - 0.5 g
- Moderate to strong: 1.5 - 5 g maximum
SHORT-TERM EFFECTS
The effects are similar to those produced by LSD.
- With small doses: stimulating and toning.
- With medium doses: mildly hallucinogenic and imagination stimulating.
- With strong doses: highly hallucinogenic and very psychedelic; the environment is often perceived as in a dream; immersed in a foreign universe; a strong awakening of self-awareness and strong feelings of communication with nature.
Start of the product effect: on average after about 30 minutes.
Duration of the effects: 3 to 12 hours, depending on the type and preparation of it (also from the user's size, weight, age, etc.).
Effects include feelings of mental and physical relaxation, feeling of sluggishness, feeling of detachment from reality, and sometimes, feelings of physical heaviness or, conversely, of lightness. Also, mood changes are produced.
Psychedelic substances profoundly modify perception and provoke a dream state rich in hallucinations.
If everything goes well, users laugh a lot.
Immediate side effects related to the consumption of mushrooms may provoke: dizziness, abdominal pain, dryness of the tongue and mouth, nausea, discomfort, confusion, anxiety with loss of control, states of excitement and delirium, respiratory crises, disturbances of movement and balance.
Also, there are signs of a sensation of time slowing down, facial flushing, frequent breathing, a sensation of detachment from the body (depersonalization), a feeling of unreality, and a lack of concentration capacity.
On the physical level, there is an increase in heart rate, arterial hypertension (increase in blood pressure), dilation of the pupils, hypothermia (decrease in body temperature), and hyperglycemia (increase in blood sugar level).
LONG-TERM EFFECTS
In cases of strong external emotional stimuli, panic attacks may occur.
Consuming psilocybin can lead to the emergence of latent (hidden) psychiatric disorders.
There is a phenomenon known as flashback: symptoms may reappear even without drug intake. If psilocybin is abused, these symptoms can become persistent (chronic visual disorder).
Over time, tolerance to psilocybin develops, which compels you to increase the doses. An overdose can put your life at risk (fatal intoxication) and make it easier to lose your normal state.
If you have a prior psychiatric history, or if you have a strong consumption of these drugs, the use of hallucinogenic mushrooms promotes anxiety, depression, disorientation, and the inability to distinguish reality from illusion. Psychiatric disorders (anxiety, phobias, confused states) may become irreversible.
PCP - PHENCYCLIDINE
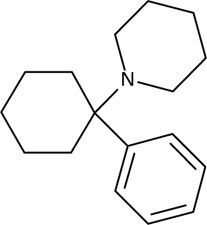
2-dimensional chemical structure of PCP
3-dimensional chemical structure of PCP
- Formula: C17H25N
- Molar mass: 243.387 g/mol
- Routes of administration: Smoked, snorted, taken orally
- Category: Hallucinogenic stimulant/disruptor
- Street names: Bella (mess), “angel dust”, peace pill, fairy dust, crystal.
- Appearance: Often found in liquid form, as powder, tablets, capsules, or paste. In reality, its appearance varies as it is mixed with a large number of drugs like ecstasy, mescaline, and ketamine. It is often sold under another name.



Phencyclidine is a complex abbreviation of the chemical name phenyl-cyclo-hexyl-piperidine (phenylcyclohexylpiperidine) and is generally known as PCP, based on the initials of the name. It is also known by the name “angel dust” and other street names.
It is a drug with recreational and dissociative (detached from reality) activity and, on the other hand, has been used as an anesthetic, having hallucinogenic and neurotoxic effects.
In its chemical structure, PCP is a derivative of arylcyclohexylamine and, in pharmacology, it is a member of the family of dissociative anesthetics.
HISTORY
Synthesized in 1926, it was first licensed in 1952 by the pharmaceutical company Parke-Davis and was marketed under the name (brand) Sernyl (derived from serenity - peace) as an anesthetic.
Due to its long half-life and harmful side effects such as: hallucinations, mania, delirium, and disorientations, it was removed from the market in 1965, to be limited for use in veterinary medicine as an anesthetic and sedative for animals. Today, veterinarians no longer use it, and it is manufactured only in clandestine laboratories.
In the early 1970s, the first drug addicts who used this substance appeared, easily synthesized in a clandestine laboratory. The multiplication of drug addiction cases led, in 1978, to the prohibition of its industrial manufacturing.
MODES OF CONSUMPTION
PCP can be snorted, smoked, swallowed, or injected.
In its pure form (“free base”), PCP is a yellow oil (usually dissolved in petroleum ether, in diethyl ether, or in tetrahydrofuran).
After treatment with hydrogen chloride gas, or with isopropyl alcohol saturated with hydrochloric acid, this oil precipitates into crystals or white to reddish powder (PCP hydrochloride). In this salt form, PCP can be snorted, depending on its purity. However, most PCP on the illegal market often contains a number of impurities, as a result of improvised production, causing the color to range from reddish to brown and the composition to range from powder to a gummy mass (paste).
Impurities can range from a reaction piperidine and other precursors, to carcinogens such as benzene and cyanide-like compounds such as PCC (piperidinocyclohexane carbonitrile).
The term "embalming fluid" is often used to refer to PCP in liquid form, in which a cigarette is dipped, to be consumed by smoking, usually known as "boat" or "water".
Often PCP is stuck on hand like LSD, like THC, like mescaline or another drug.
Samples analyzed by various toxicology laboratories contain 1.3 mg to 81 mg PCP, while 1 mg to 5 mg are sufficient to provoke euphoria.


SHORT-TERM EFFECTS
The effects last between 3 and 18 hours.
When taken in small doses, a pleasant state of intoxication is produced, a sense of detachment from reality, perceptual distortions, and difficulty in concentration and communication.
With higher doses, users can become very withdrawn, paranoid, frightened, aggressive, and passive. "Bad trips" are more common with PCP when it is combined with other drugs.
Consuming an overdose can provoke convulsions, coma, and death. The confusion that comes from drug use can also cause accidental death.
LONG-TERM EFFECTS
In the long term, there is a possibility of relapse, speech problems can increase, there may be depression, anxiety, or worsened psychological effects.
Regular consumption leads to tolerance. Chronic users can become psychologically dependent. PCP does not lead to physical dependency.
PCP disrupts the body's natural reflexes such as heartbeats. Indeed, under the effect of PCP, the heart can lose its reflex to beat.
PCP can cause irreparable damage to the brain, such as the loss of ability to urinate and to defecate naturally.
PCP can make you aggressive and disrupt short-term and long-term memory. It can cause psychological disorders or lead to suicide, during regular use or overdose.
KETAMINE
2-dimensional chemical structure of ketamine
3-dimensional chemical structure of ketamine
- Appearance: Crystalline powder or clear solution
- Formula: C13H16ClNO [Isomer]
- Molar mass: 237.725 ± 0.014 g/mol
- Therapeutic class: Analgesic, General Anesthetic
- Routes of administration: Oral, intravenous, intramuscular
- Methods of consumption: Snorting, injection
- Psychotropic character: Hallucinogenic
- Other street names: Keta, Ket, K, Special K, Spe, Angel Dust, Keti, Kit kat, Vitamin K (not to be confused with Vitamin K)

Ketamine is a fast-acting painkiller and anesthetic, primarily used in veterinary surgery. It is also used to a lesser extent in human medicine. A close relative of phencyclidine (PCP), ketamine belongs to the central nervous system disruptors.
The desired effect is “soul melting”, a near-death experience that gives the sensation of “soul detachment from the body”, and “floating above your body”. This “separation” results in deep hallucinations and feelings of living in another reality. For this reason, the use of ketamine in humans, for medical purposes, is done at the same time as another medication to prevent hallucinations.
HISTORY
Ketamine was first synthesized in 1962 by Calvin Stevens, a consultant for the American pharmaceutical laboratory Parke Davis, who was conducting scientific research on alpha-hydroxyimine modifications.
After promising preclinical studies in animals, ketamine was tested in humans, on prisoners, in 1964. These tests demonstrated that the short duration of ketamine’s action and its reduced behavioral toxicity made it a more favorable choice than PCP as a dissociative anesthetic.
After approval by the FDA in 1970, ketamine anesthesia was first given to American soldiers during the Vietnam War.
Non-medical (recreational) use of ketamine began on the West Coast of the United States in the early 1970s. Early use is documented in underground literature such as “The Fabulous Furry Freak Brothers”.
Ketamine has been used in psychiatric scientific research and other academic scientific research during the 1970s, peaking in 1978 with the publication of psychiatrist John Lilly’s “The Scientist”, and that of Marcia Moore and Howard Alltounian, “Journeys into the Bright World”, which documented the unusual phenomenology of ketamine intoxication.
The trend of recreational use of ketamine increased until the end of the 20th century, especially in the context of rave parties. However, its emergence as a “club drug” differs from other club drugs (e.g., from MDMA) due to its anesthetic properties at higher doses (e.g., slurred speech, immobilization).
The rise of ketamine in dance culture was faster in Hong Kong in the late 1990s.
Before becoming a controlled substance by the federal government in the United States, in 1999, ketamine was available as a pharmaceutical preparation for recreational purposes and as a pure powder, sold in large quantities by domestic chemical supply companies. Today, most ketamine for non-medical use originates from China and India.
Due to its ability to cause confusion and amnesic effects, ketamine can make users vulnerable to rape, hence it is also called a "rape drug".
METHODS OF CONSUMPTION
Ketamine manufactured for medical purposes is sold in liquid form.
In other cases, it is generally transformed into a white powder before being sold as an illegal drug. The powder is snorted, mixed with drinks, or smoked with marijuana or tobacco.
The liquid is added to drinks or usually injected into a muscle, as injection into a vein typically results in a loss of consciousness.
Only veterinarians and doctors can legally procure ketamine. It is then stolen or diverted and sold illegally on the streets or in clubs for recreational use.

SHORT-TERM EFFECTS
The effects of ketamine are usually felt 10 minutes after consumption.
When the dose of ketamine is small, users experience a sense of drowsiness, numbing or withdrawal into themselves. They may find it difficult to think clearly, feel confused, and have a different perception of time and their body.
If they take a stronger dose, their discussions may be incomprehensible, they may forget who they are and where they are, they hesitate when walking (taking steps), notice an acceleration of heart rate and have difficulty breathing.
A very strong dose of ketamine risks leading to a loss of sensation.
The effects of ketamine last about an hour. Some users risk being in a state of depression and anxiety, suffering from memory loss, or returning to their original state much later than the drug's effects have disappeared.
Users report feeling drunk, dizzy, and having a numb sensation in their body. Visual experiences include a blurred vision, the impression of seeing "trails" and "traveling in space" and strong and terrifying hallucinations. Some people experience a sense of relief and "melting into the body" or a "near-death experience".
Ketamine users risk putting their life in danger if this drug is not administered by health professionals in a medical setting. Like all anesthetics, ketamine prevents the user from feeling pain. Therefore, if the user is injured, they risk not noticing it.
Depending on the dose taken, individuals under its influence risk having difficulty standing or expressing themselves, which increases the risk of bodily injuries.
As with other anesthetics, ketamine can cause vomiting. On the other hand, the fact of consuming food or drinks before taking ketamine increases the risk of choking on vomit.
Taken in higher doses, ketamine can depress the CNS, thus causing poor oxygenation of the brain, heart, and other muscles, which can be fatal.
Combining ketamine with alcohol or sedatives risks death.
A user who regularly takes ketamine develops a tolerance to the effects of this drug.
Some reports provide the condition of typical jaundice symptoms when users stop taking ketamine.
The long-term effects are not known, because there is very little scientific research on the non-medical long-term use of this drug.
GHB - GAMMA-HYDROXYBUTYRATE

2-dimensional chemical structure of GHB
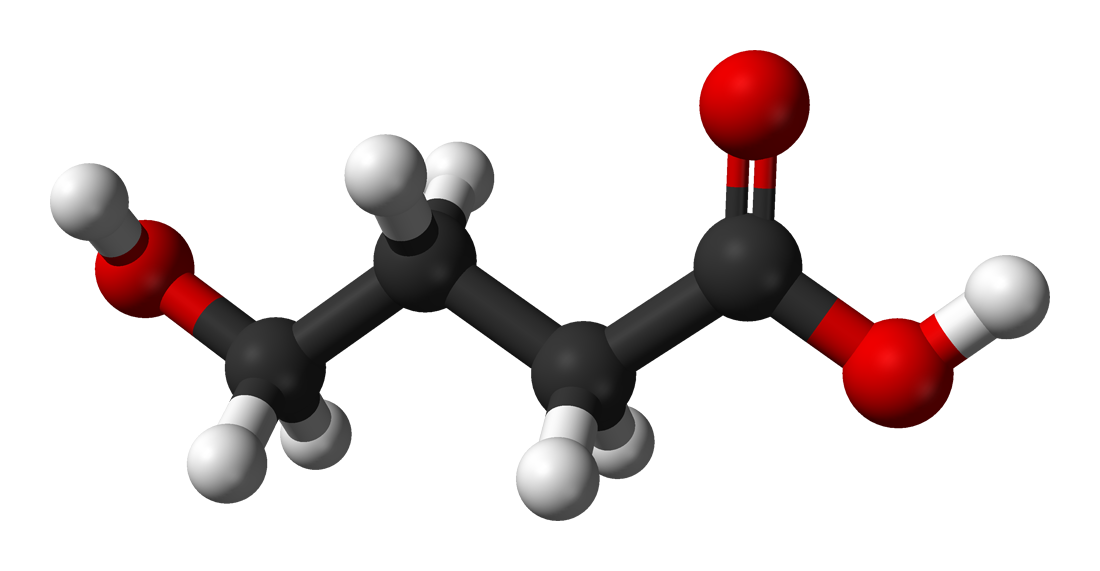
3-dimensional chemical structure of GHB
- Appearance: Colorless and odorless liquid
- Chemical formula: C4H8O3 [Isomers]
- Molar mass: 104.1045 ± 0.0047 g/mol
- Fusion temperature: - 17 °C
- Melting temperature: 178 to 180 °C (decomposition)
- Category: Depressant
- Method of consumption: Swallowing
- Other names: Gamma-hydroxybutyrate, Gamma-oh, GHB, G, GBH, Georgia Home Boy, Grievous Bodily Harm, Liquid X, Liquid E, Liquid Ecstasy, Blue Verve, Scoop, Fantasy, etc.
γ-hydroxybutyrate (GHB), also known under the name 4-hydroxybutanoic acid and sodium oxybate, is a natural substance discovered in the central nervous system, in wine, in veal, in citrus fruits and almost in all animal meats in small quantities.
GHB, a sodium salt, known under the name sodium oxybate, is sold by Jazz Pharmaceuticals under the name Xyrem to treat cataplexy and excessive daytime sleepiness in patients affected by narcolepsy (sleep disorder).
GHB is a psychoactive depressant, used for medical purposes or for recreational purposes. GHB is a chemical structure very similar to that of a neurotransmitter, GABA.
GHB has been used in medical settings as a common anesthetic, to treat conditions such as insomnia, clinical depression, narcolepsy, alcoholism, and to improve athletic performance. GHB is also used as a hallucinogenic substance (illegally, according to many jurisdictions) or as a "rape drug". It is a natural product in human body cells and is structurally related to beta-hydroxybutyrate of ketone bodies (like whale's). Being a supplement/drug, it is often used in the form of a salt. GHB is also a product of the fermentation process, and thus, is found in small quantities in some beers and wines.
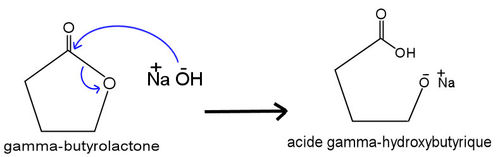
GHB is synthesized starting from γ-butyrolactone (GBL), adding sodium hydroxide (NaOH) in ethanol or in water.
In the brains of mammals, GHB is synthesized starting from gamma-aminobutyric acid (GABA).
GHB is considered to be less toxic than GBL for the same effects.
As a drug, GHB is most commonly used in the form of a chemical salt (Na-GHB or K-GHB) and is most often sold in liquid form (crystal), but sometimes in powder form.

GHB temporarily prevents the diffusion of dopamine and thereby increases its concentration in the synapse.
GHB stimulates the production of growth hormone from the pituitary gland.
GHB acts on endorphins giving GHB sedative (calming) and anesthetic properties.
GHB acts especially in the septum and hippocampus through the locus coeruleus. GHB partially administers behaviors of alarm, fear, anxiety, and habit.
Also, it is GHB that puts the body's muscles in a state of deep relaxation (even paralysis) during dreaming or sleep.
HISTORY
The synthesis of the chemical substance GHB was initially signaled in the year 1874 by Alexander Zaitsev, but, initially, the primary scientific research on its use in humans was conducted in the 1960s, in the Balmer & CO laboratories, by Dr. Henri Laborit in the study of the neurotransmitter GABA.
GHB quickly found a wide range of use due to its minimal side effects and short duration of action, considering the risks presented by its combination with alcohol and other depressants of the central nervous system (CNS).
GHB has been widely used in France, Italy, and other European countries for several decades as a birthing agent and an anesthetic during childbirth.
Problems with its potential for abuse and the development of new drugs have led to a recent decrease in the legal medical use of GHB.
Sodium oxybate has been used as a general anesthetic and as a hypnotic in the treatment of insomnia, especially in the treatment of narcolepsy under the brand name Xyrem©, or, in France, under the name Gamma-OH©.
For many years, in the Netherlands (Holland, Belgium, etc), GHB could be purchased as an aphrodisiac and euphoriant in a «smart shop».
Today, the only common medical applications of GHB are in the treatment of narcolepsy and, less frequently, in the treatment of alcoholism.
Starting from the 1990s, GHB has been used as a «date rape drug», because it dissolves easily in alcohol (very mild taste) and, at high doses, induces a hypnotic state and amnesia (memory disturbances). This use is considered very widespread in the USA and Canada, where the necessary products for the manufacture of GHB are freely available for sale.
In the 1980s, those who were into "body building" used GHB as a dietary supplement, because it stimulates growth hormones. This use was banned over the course of the 1990s.
Until 1998, GHB was relatively available on the Internet.
According to the INCB (International Narcotics Control Board), like most "synthetic drugs", production primarily occurs near consumption areas, through the operation of mobile laboratories.
In the USA, its sale to the public was banned in 2000, but since 2002 GHB has been advised in the treatment of narcolepsy accompanied by cataplexy.
In most countries, GHB is classified according to the 1971 convention on psychotropic substances.
SHORT-TERM EFFECTS
The effects of GHB manifest quickly, within 10 to 15 minutes on average, and last for 45 to 90 minutes.
The desired effects include:
- Muscle relaxation;
- Reduction and mitigation of anxiety;
- Euphoria.
The short-term effects are:
- Slowing of heart rate and respiration;
- Hypotension;
- Loss of coordination and balance;
- Confusion, dizziness, vomiting;
- Amnesia;
- Drowsiness leading to loss of consciousness (fainting), or coma.
LONG-TERM EFFECTS
Excessive and prolonged use leads to tolerance and physical dependence.
Withdrawal symptoms (detoxification, abstinence) are rapid and include anxiety, insomnia, disturbances, irritation, sensitivity to external stimuli (noise, light, touch), tachycardia, and muscle cramps. These withdrawal symptoms appear from 1 to 6 hours after the last consumption and disappear after 2 to 21 days depending on the dependency.
The only known cases of GHB overdose in humans are related to a mixture of GHB with alcohol, a mixture often encountered when GHB is used as a recreational drug.
Since the effects of alcohol and GHB are synergistic, combining them in large amounts turns GHB de facto very toxic.
GBL - GAMMA-BUTYROLACTONE
GENERAL INFORMATION
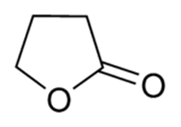
2-dimensional chemical structure of GBL
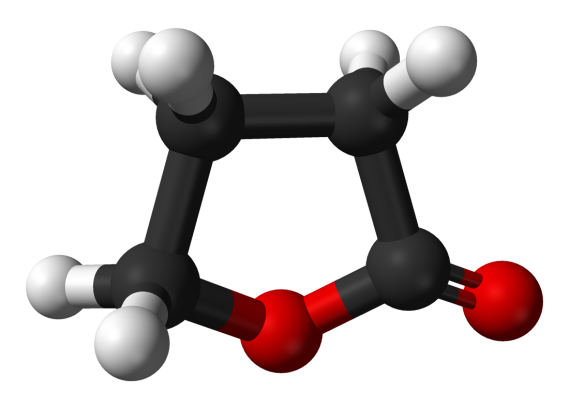 3-dimensional chemical structure of GBL
3-dimensional chemical structure of GBL
Gamma-butyrolactone (GBL) is a precursor to gamma-hydroxybutyric acid (GHB). It is used in the industry as a solvent for paint, for nail varnishes where its highly acidic properties dissolve plastics, clean, and bleach varnishes. Since gamma-butyrolactone (GBL) is transformed into GHB in the human body, what has been said above for GHB also applies to GBL.
